Abstract
Introduction: Sickle cell disease (SCD) is a genetic disorder in which hypoxia produces polymerization of sickle hemoglobin (HbS) triggering multiple downstream effects of red cell distortion (sickling), hemolysis, occlusion of blood flow, and inflammation. Injury from SCD starts at birth and accumulates over a patient's lifetime causing significant end-organ damage, as well as leading to fatigue, pain and other clinical complications that are under-recognized, under-treated, and associated with early death. Patients with the homozygous mutation (HbSS), exhibit the severe form of SCD. All individuals with SCD are at increased risk of infection; though those under 2 years of age are at a higher risk (Brousse et al, Br J Haematol 2014). Individuals with SCD are also at increased risk of stroke and retinopathy. The risk for ischemic stroke in SCD is highest in patients under 21 years of age. In light of the potential serious complications of SCD, clinical guidelines for pediatric SCD patients recommend prophylactic penicillin use (age 2-5), annual screening for stroke with trans-cranial doppler (TCD) imaging (age 2-16), and annual ophthalmology exams to assess for retinopathy (age ≥10). As there is limited real world data on the level of implementation of these NHLBI-based recommendations, this study aims to describe the use of penicillin, TCD screening, and ophthalmology care in a large cohort of children with HbSS disease.
Methods: The Truven MarketScan® Commercial and Medicaid administrative claims databases were used to identify cohorts of United States patients who were age 2-16 at first indication of HbSS (1 inpatient or 2 outpatient claims with ICD-9 282.62, 282.61) recorded in each calendar year from 2009 to 2014; patients may have been diagnosed previously. In each cohort, patients were required to have medical and pharmacy benefits for the calendar year in which they were identified and for 12 months prior to their first recorded HbSS indication. Patients undergoing chronic red blood cell transfusion therapy (defined here as ≥ 9 in a calendar year) were excluded. Prior year utilization of penicillin, TCDs, and ophthalmologist visits (proxy for retinopathy screening) was measured for each cohort. Results are reported by payer (Commercial, Medicaid) and age group, and presented as ranges across the annual cohorts.
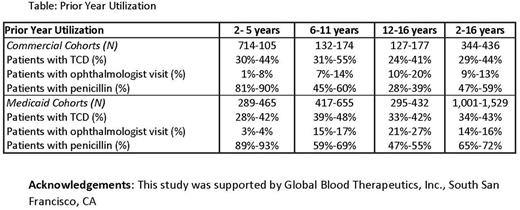
Results: Annual cohorts included 344-436 Commercial and 1,001-1,529 Medicaid HbSS patients. In both Commercial and Medicaid cohorts, the mean age was 8-9 years, and 50% of patients were female. The majority of patients resided in urban areas (89%-95% in Commercial cohorts; 78%-85% in Medicaid cohorts).
In both the Commercial and Medicaid cohorts, fewer than half of all patients had received an TCD scan in the previous 12 months. Similar proportions of patients with a TCD scan (24%-55%) were seen across all age groups for both payers. The proportion of patients with TCDs was highest among children 6-11 years old among both payers (Table).
For both payers, the use of ophthalmologist visits increased as patients aged, and while patients 12-16 years old had the highest proportion with an ophthalmologist visit in both payer populations (Table), the overall implementation remained low (only 10% - 27% in this age group had an ophthalmologist visit).
In contrast to the low use of TCD and ophthalmology visits, the use of penicillin was highest in the 2-5 age group at > 80% use in any given year for both payers.
No substantial differences were found in the utilization patterns of urban and non-urban patients in either payer population.
Conclusions: While our data demonstrated high use of penicillin in the 2-5 year old population, consistent with guidelines, there is an opportunity to improve implementation of other guideline-based recommended screening necessary to prevent other serious SCD complications. For example, TCD screening can identify children at risk of SCD-related stroke in order to initiate preventative therapies. As stroke can lead to devastating cognitive impairment and other disabilities (e.g., hemiplegia, sensory impairments, epilepsy), it is especially important that patients are appropriately screened. Further research to understand potential barriers to proper screening, and to evaluate strategies to improve awareness, adherence and implementation of recommended screenings in children with SCD is warranted.
Kanter: Apopharma: Research Funding; GBT: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; MUSC: Other: The site PI for sponsored research conducted at MUSC who receives funds from: Novartis, bluebird bio, GBT, Sancillo, Apopharma, Pfizer; American Society of Hematology (Sickle Cell Disease Guideline Panel): Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; NHLBI (sickle cell disease research advisory committee): Membership on an entity's Board of Directors or advisory committees, Research Funding; Sancillo: Research Funding; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Dampier: Pfizer, Lilly, Novartis, Sancilio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Agodoa: Global Blood Therapeutics, Inc.: Employment, Equity Ownership. Howard: Global Blood Therapeutics: Employment, Equity Ownership. Wade: Truven Health Analytics, an IBM company which received project funding from Global Blood Therapeutics: Consultancy. Noxon: Truven Health Analytics, an IBM company which received project funding from Global Blood Therapeutics: Employment. Ballas: Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal